Genetic Testing Processing and Procedures at IDNs: What Manufacturers Need to Know
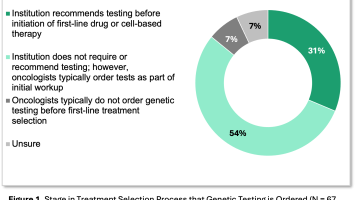
Our latest data show that more than 85% of oncologists are ordering genetic tests early in the treatment process (Figure 1). More specifically, 31% of IDN oncologists indicate that IDNs recommend testing before initiation of a first-line drug or cell-based therapy, and another 54% of IDN oncologists order tests as part of the initial workup even if the IDN has no specific recommendations or requirements around genetic testing. For commonly ordered genetic tests, IDN oncologists indicate that there are limited IDN, lab, or payer reimbursement barriers to ordering. Payers and IDNs generally both align with National Comprehensive Cancer Network (NCCN) Guidelines for major tumor types.

Barriers to Genetic Testing Remain in Oncology
- Genetic Testing Varies By Tumor Type: Oncologists routinely order genetic panels for high-incidence cancers, such as breast and lung. However, for tumor types with smaller patient populations or for genetic mutations with more recently approved targeted therapies, testing is less consistent.
- Barriers Remain for Rare Biomarkers: For less common mutations or newer biomarker targets, genetic testing can still delay access to care. If a test is not part of an IDN’s standard panel, oncologists may encounter internal delays and face payer prior authorization or reimbursement hurdles.
- External Labs Add Complexity: When IDN oncologists need to order tests from external labs, oncologists must often navigate additional steps, including delays tied to internal IDN workflows and approvals or additional documentation with payers.
Oncology Manufacturer Recommendations
- Understand How Individual IDNs Approach Genetic Testing: Manufacturers with brands targeting specific mutations should closely monitor test selection and frequency for key IDN customers. It is essential to understand whether oncologists typically use broad genomic panels or narrower assays and whether your brand’s targeted biomarker is consistently included for each tumor type. If not, targeted IDN engagement by medical teams may be needed to address testing gaps that limit brand access.
- Streamline Coordination With External Labs: If a manufacturers’ therapy relies on a test performed by an external lab, collaboration with that vendor is critical. Manufacturers should ensure the lab is approved across major IDNs and accepted by key payer partners. Streamlining the ordering and approval process can help reduce treatment delays and support brand access.
Additional information can be found in the 2025 MARIN Oncology Trend Report and genetic testing practices by specific IDN account will also be outlined in the 2025 MARIN IDN Dataset to be released at the end of this month. Want to know more about this report or the upcoming dataset? I’d love to connect: ebijesse@hmplgobal.com.