Oncology's Prior Authorization Problem: Providers Continue to Face Significant Barriers
Prior authorization (PA) requirements continue to be one of the biggest hurdles for oncology providers—impacting access, increasing administrative burdens, and frustrating both patients and physicians. Read our latest analysis to learn why.
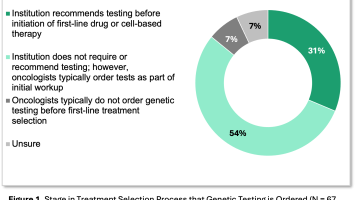
Prior authorization (PA) requirements are the most commonly deployed payer utilization management (UM) tool for oncology drugs. Our ongoing conversations with providers indicate that PA requirements impact most oncology drugs and continue to be more onerous for providers and patients. These frustrations are substantiated by our 2024 Oncology Customer Segment reports, including the Integrated Delivery Network (IDN), Community, and Payer reports. Our research found that PAs are initially denied 24% of the time at IDNs and 20% of the time at community oncology practices. Payers generally report similar levels of initial oncology PA denials, at ~20%.
While initial PA denial rates are consistent across customer segments, the rate of PA appeals after an initial denial differ across customer segments. IDNs are more likely than community oncology practices to appeal PA denials, with 41% of denied PAs appealed at IDNs compared to 34% at community oncology practices.
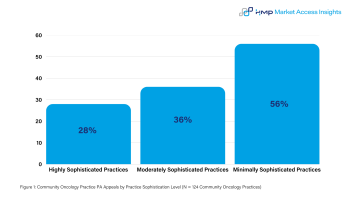
Another important distinction as seen in the figure below is that PA denial appeals submissions are inversely correlated with practice sophistication. Less sophisticated community practices are more likely to appeal PA denials than highly sophisticated community oncology practices. Minimally sophisticated community oncology practices appeal 56% of PA denials, while highly sophisticated practices appeal just 28% of PA denials. This trend also holds true from IDNs where the least sophisticated and integrated IDNs are the most likely to appeal PA denials.
Key Takeaways
- Oncology drug manufacturers should expect ongoing PA requirements for products and must proactively work with payers to minimize requirements and limit PA denials, (eg, provide new clinical trial data or RWE updates).
- If a manufacturer’s brand is frequently denied during the PA process, it is important to collaborate with sophisticated community oncology practices to facilitate and provide the relevant data for a standard process for appeals.
- For less sophisticated community oncology practices and IDNs, working with individual providers or sites to build brand awareness and offering resources to support prior authorizations will likely continue to be a successful strategy to support PA appeals.
For more information on this, keep an eye out for our first 2025 Landscape Report to be released at the end of this month. The report will include additional cross customer segment analyses and insights for manufacturers.