What's Going On with Oncology Pathways? Here's What You Need to Know
November 30, 2023
Welcome to the November 2023 edition of our Monthly Insight Series. This month we are highlighting a few key findings from our recently published report on Oncology Clinical Pathways.
Oncology pathways have been a hot topic of discussion for several years. Perceptions, even definitions, of pathways are highly variable, and their true impact on oncologists’ treatment selection is nuanced. This week, Market Access Insights published the first of two deliverables for our inaugural pathway report, and we want to share a few topline findings!

- A sizeable minority of providers and payers are using or experimenting with oncology clinical pathways. Payer and provider interest in pathways will increase:
- Pathways provide a very efficient channel for academic experts to share best practices with non-academicians who struggle to remain up to date with rapidly expanding sets of treatment options.
- Reducing variation in treatment selections helps to improve clinical quality for providers and cost predictability for payers and at-risk providers.
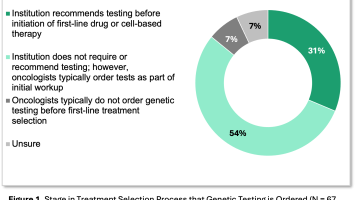
- While NCCN continues to be referenced as a source for several pathway programs, the largest payer pathways usually include fewer than 50% of NCCN’s preferred regimens
- Pathways generally encourage oncologists to select from 1-3 options, rather than the 6-10 that are commonly listed as “preferred” by NCCN’s guidelines.
- The high congruence with NCCN means that many treatment choices appear to be “on-pathway” even in cases where the prescribing oncologist is unaware of a pathway; MAI terms this situation “unconscious compliance.”
- Pathway developers vary in their assessment processes but universally emphasize relying upon published clinical trial data
- Manufacturers may submit data to many developers, but the developers may not rely on or review the submissions.
- Exceptions include McKesson’s interest in total cost of care and other HEOR studies, and ClinicalPath’s occasional review of submitted data.